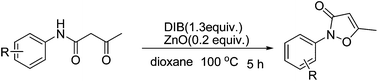
Direct construction of 5-methyl-2-phenylisoxazol-3(2H)-ones via hypervalent iodine mediated sequential tandem oxidative cyclization of 3-oxo-N-phenylbutanamides catalyzed by zinc oxide (ZnO)†
Abstract
A sequential oxidative tandem cyclization reaction mediated by a combination of

 Please wait while we load your content...
Please wait while we load your content...