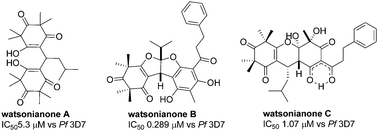
Three new β-triketones, watsonianones A–C, and the known compound corymbone B were isolated from the flowers of the Australian eucalypt Corymbia watsoniana. Watsonianone A is the first naturally occurring methylene bridged bis-tetramethylcyclohexatrione, watsonianone B is only the fourth fused bisfurano β-triketone and watsonianone C is the first 4,4a,9,9a-tetrahydro-2H-xanthene-1,3,5,7(6H,8H)-tetraone to be reported in the literature. MS and NMR analysis established the structures of the new compounds. All three new compounds showed anti-plasmodial activity against chloroquine resistant (Dd2) and sensitive strains (3D7) of the parasite, Plasmodium falciparum, responsible for malarial infections. Watsonianone B was the most potent inhibitor (IC50 0.289 μM vs. Pf 3D7) demonstrating significant selectivity against the human cell line, HEK 293 (>400 ×). Stage specificity studies indicate that watsonianone B is predominantly active against young ring stages of P. falciparum.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...