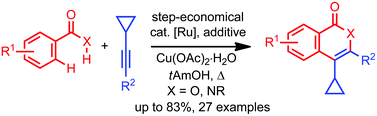
Ruthenium-catalyzed C–H/O–H and C–H/N–H bond functionalizations: oxidative annulations of cyclopropyl-substituted alkynes†
Abstract
The chemical behavior of
- This article is part of the themed collection: Celebrating the 150th anniversary of the German Chemical Society

 Please wait while we load your content...
Please wait while we load your content...