Atomistic modelling of CVD synthesis of carbon nanotubes and graphene
Abstract
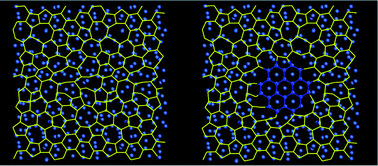
We discuss the synthesis of
* Corresponding authors
a
Department of Materials Science and Metallurgy, University of Cambridge, Pembroke Street, Cambridge, UK
E-mail:
jae1001@cam.ac.uk
b
Department of Materials Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
E-mail:
shibuta@material.t.u-tokyo.ac.jp
c
Laboratoire d'Etudes des Microstructures, ONERA-CNRS, BP 72, 92322 Châtillon Cedex, France
E-mail:
hakim.amara@onera.fr
d
Aix-Marseille Université, CNRS, CINaM UMR 7325, 13288 Marseille, France
E-mail:
xtof@cinam.univ-mrs.fr
e
Department of Chemistry, PLASMANT Research Group, University of Antwerp, Universiteitsplein 1, B-2610 Wilrijk-Antwerp, Belgium
E-mail:
erik.neyts@ua.ac.be
We discuss the synthesis of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
J. A. Elliott, Y. Shibuta, H. Amara, C. Bichara and E. C. Neyts, Nanoscale, 2013, 5, 6662 DOI: 10.1039/C3NR01925J
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. You can use material from this article in other publications without requesting further permissions from the RSC, provided that the correct acknowledgement is given.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
