Electronic subshell splitting controls the atomic structure of charged and neutral silver clusters†
Abstract
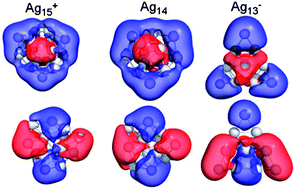
Theoretical studies on the atomic and electronic structure of Agn−,0,+ clusters containing up to 15 atoms have been carried out using a gradient corrected density functional formalism. Our studies indicate that the atomic structures of the clusters undergo a transition from spherical to prolate to oblate shapes as a function of the number of valence electrons. Clusters with a valence count of 8 are shown to be highly stable and their stability is rooted in the filled 1S, 1P shells within a confined spherical nearly free electron gas. Clusters with 14 valence electrons are also shown to be highly stable, but their stability arises due to oblate geometrical distortions that lead to a splitting of the 1D shell with a large HOMO–LUMO gap. The large splitting is shown to account for the observed unusual inertness of these clusters in reactivity with oxygen. The theoretical findings are compared with the observed peaks in the photodetachment spectrum of the anionic species.

 Please wait while we load your content...
Please wait while we load your content...