Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis†
Abstract
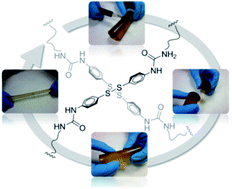
Aromatic disulfide metathesis has been reported as one of the very few dynamic covalent chemistries undergone at room-temperature. Here, bis(4-aminophenyl) disulfide is effectively used as a dynamic crosslinker for the design of self-healing poly(urea–urethane) elastomers, which show quantitative healing efficiency at room-temperature, without the need for any catalyst or external intervention.
- This article is part of the themed collection: Materials Horizons 10th anniversary regional spotlight collection: Europe

 Please wait while we load your content...
Please wait while we load your content...