Design and synthesis of a novel series of cyclohexyloxy-pyridyl derivatives as inhibitors of diacylglycerol acyl transferase 1†
Abstract
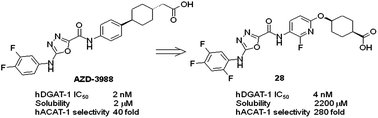
A novel series of potent diacylglycerol acyl transferase 1 inhibitors was developed from the clinical candidate AZD3988. Replacement of the phenyl cyclohexyl-ethanoate side chain with substituted oxy-linked side chains to introduce changes in shape and polarity, reduce lipophilicity and mask the hydrogen bond donors with internal hydrogen bond acceptors led to improvements in solubility, unbound clearance and excellent selectivity over the related enzyme acyl-coenzyme A:cholesterol acyltransferase 1. A comparison of the small molecule crystal structures of compound 4 and compound 28 is described. Compounds in this series have good ADMET properties and provide an exposure-dependent decrease in circulating plasma triglyceride levels in a rat oral lipid tolerance test.

 Please wait while we load your content...
Please wait while we load your content...