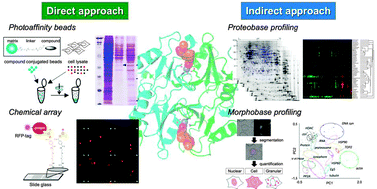
Recently, a phenotypic approach—screens that assess the effects of compounds on cells, tissues, or whole organisms—has been reconsidered and reintroduced as a complementary strategy of a target-based approach for drug discovery. Although the finding of novel bioactive compounds from large chemical libraries has become routine, the identification of their molecular targets is still a time-consuming and difficult process, making this step rate-limiting in drug development. In the last decade, we and other researchers have amassed a large amount of phenotypic data through progress in omics research and advances in instrumentation. Accordingly, the profiling methodologies using these datasets expertly have emerged to identify and validate specific molecular targets of drug candidates, attaining some progress in current drug discovery (e.g., eribulin). In the case of a compound that shows an unprecedented phenotype likely by inhibiting a first-in-class target, however, such phenotypic profiling is invalid. Under the circumstances, a photo-crosslinking affinity approach should be beneficial. In this review, we describe and summarize recent progress in both affinity-based (direct) and phenotypic profiling (indirect) approaches for chemical biology target identification.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...