Ionic shield for polysulfides towards highly-stable lithium–sulfur batteries†
Abstract
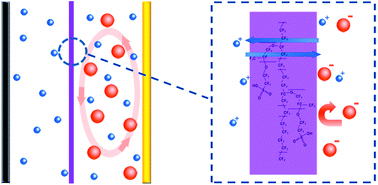
Lithium–sulfur batteries attract great attention due to their high energy density, while their real applications are still hindered by the rapid capacity degradation. Despite great efforts devoted to solving the polysulfide shuttle between the cathode and anode electrodes, it remains a serious challenge to build highly-stable lithium–sulfur batteries. Herein we demonstrate a strategy of introducing an ion selective membrane to improve the stability and coulombic efficiency of lithium–sulfur batteries. The sulfonate-ended perfluoroalkyl ether groups on the ionic separators are connected by pores or channels that are around several nanometers in size. These SO3− groups-coated channels allow ion hopping of positively charged species (Li+) but reject hopping of negative ions, such as polysulfide anions (Sn2−) in this specific case due to the coulombic interactions. Consequently, this cation permselective membrane acts as an electrostatic shield for polysulfide anions, and confines the polysulfides on the cathode side. An ultra-low decay rate of 0.08% per cycle is achieved within the initial 500 cycles for the membrane developed in this work, which is less than half that of the routine membranes. Such an ion selective membrane is versatile for various electrodes and working conditions, which is promising for the construction of high performance batteries.

 Please wait while we load your content...
Please wait while we load your content...