Direct prediction of the desalination performance of porous carbon electrodes for capacitive deionization†
Abstract
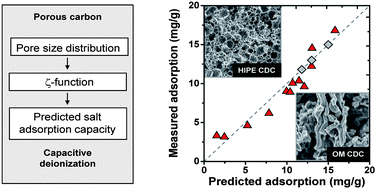
Desalination by capacitive deionization (CDI) is an emerging technology for the energy- and cost-efficient removal of ions from water by electrosorption in charged porous carbon electrodes. A variety of carbon materials, including activated carbons, templated carbons, carbon aerogels, and carbon nanotubes, have been studied as electrode materials for CDI. Using carbide-derived carbons (CDCs) with precisely tailored pore size distributions (PSD) of micro- and mesopores, we studied experimentally and theoretically the effect of pore architecture on salt electrosorption capacity and salt removal rate. Of the reported CDC-materials, ordered mesoporous silicon carbide-derived carbon (OM SiC-CDC), with a bimodal distribution of pore sizes at 1 and 4 nm, shows the highest salt electrosorption capacity per unit mass, namely 15.0 mg of NaCl per 1 g of porous carbon in both electrodes at a cell voltage of 1.2 V (12.8 mg per 1 g of total electrode mass). We present a method to quantify the influence of each pore size increment on desalination performance in CDI by correlating the PSD with desalination performance. We obtain a high correlation when assuming the ion adsorption capacity to increase sharply for pore sizes below one nanometer, in line with previous observations for CDI and for electrical double layer capacitors, but in contrast to the commonly held view about CDI that mesopores are required to avoid electrical double layer overlap. To quantify the dynamics of CDI, we develop a two-dimensional porous electrode modified Donnan model. For two of the tested materials, both containing a fair degree of mesopores (while the total electrode porosity is ∼95 vol%), the model describes data for the accumulation rate of charge (current) and salt accumulation very well, and also accurately reproduces the effect of an increase in electrode thickness. However, for TiC-CDC with hardly any mesopores, and with a lower total porosity, the current is underestimated. Calculation results show that a material with higher electrode porosity is not necessarily responding faster, as more porosity also implies longer transport pathways across the electrode. Our work highlights that a direct prediction of CDI performance both for equilibrium and dynamics can be achieved based on the PSD and knowledge of the geometrical structure of the electrodes.

 Please wait while we load your content...
Please wait while we load your content...