Reprogramming the glycolytic pathway for increased hydrogen production in cyanobacteria: metabolic engineering of NAD+-dependent GAPDH
Abstract
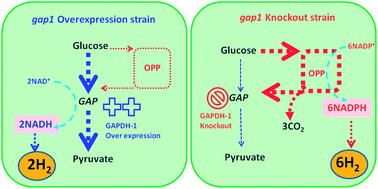
Catabolism of glycogen stored by cyanobacteria occurs during anaerobic auto-fermentation and produces a range of C1–C3 fermentation products and hydrogen via hydrogenase. We investigated both augmenting and rerouting this carbon catabolism by engineering the glycolysis pathway at the NAD+-dependent glyceraldehyde-3-phosphate dehydrogenase (GAPDH-1), its major regulation site at the nexus of two pathways (Oxidative Pentose Phosphate pathway, OPP, and glycolysis/gluconeogenesis). Null (gap1::aphII) and overexpression (gap1+) strains of Synechococcus sp. strain PCC 7002 were constructed in order to produce more NADPH (via rerouting carbon through OPP) and more NADH (via opening the glycolytic bottleneck), respectively. For gap1::aphII quantitative analyses after four days of dark auto-fermentation showed undiminished glycogen catabolism rate, significant increases of intracellular metabolites in both OPP and upper-glycolysis, decrease in lower-glycolysis intermediates, 5.7-fold increase in NADPH, 2.3-fold increase in hydrogen and 1.25-fold increase in CO2vs. wild type (WT). These changes demonstrate the expected outcome of redirection of carbon catabolism through the OPP pathway with significant stimulation of OPP product yields. The gap1+ strain exhibits a large 17% increase in accumulation of glycogen during the prior photoautotrophic growth stage (gluconeogenesis), in parallel with a 2-fold increase in the total [NAD+ + NADH] pool, foreshadowing an increased catabolic capacity. Indeed, the rate of glycogen catabolism during subsequent dark auto-fermentation increased significantly (58%) vs. WT, resulting in increases in both NADH (4.0-fold) and NADPH (2.9-fold) pools, and terminal fermentation products, hydrogen (3.0-fold) D-lactate (2.3-fold) and acetate (1.4-fold). The overall energy conversion yield over four days from catabolized glycogen to hydrogen increased from 0.6 mole of hydrogen per mole of glucose (WT) to 1.4 (gap1::aphII) and 1.1 (gap1+) under headspace accumulation conditions (without hydrogen milking). These findings demonstrate the significant potential of metabolic engineering for redirecting carbon pathways for carbohydrate catabolism and hydrogen production in cyanobacteria.

 Please wait while we load your content...
Please wait while we load your content...