Bi- and tri-metallic Rh and Ir complexes containing click derived bis- and tris-(pyrazolyl-1,2,3-triazolyl) N–N′ donor ligands and their application as catalysts for the dihydroalkoxylation of alkynes†
Abstract
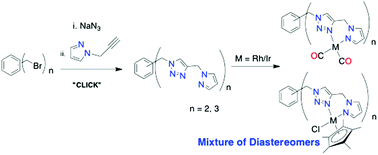
A series of bi-topic and tri-topic pyrazolyl-1,2,3-triazolyl donor ligands (1a–d; 1a–c = 1,X-bis((4-((1H-pyrazol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)methyl)benzene (X = 2, 3 and 4; o-C6H4(PyT)2, m-C6H4(PyT)2 and p-C6H4(PyT)2) and 1d = 1,3,5-tris((4-((1H-pyrazol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)methyl)benzene, 1,3,5-C6H3(PyT)3) were conveniently synthesised in ‘one pot’ reactions using the Cu(I) catalysed ‘click’ reaction. Rh(I), Ir(I), Rh(III) and Ir(III) complexes with ligands 1a–d of the general formulae C6H6−n[(PyT)M(CO)2]n[BArF4]n (M = Rh, Ir; n = 2, 3; 2a–d; 3a–d) and C6H6−n[(PyT)MCp*Cl]n[BArF4]n (M = Rh, Ir; n = 2, 3; 4a–d; 5a–d) were synthesised and fully characterised. In solution each of the bi- or tri-metallic complexes 4a–d and 5a–d exists as a mixture of two (4a–c, 5a–c) or three (4d and 5d) diastereomers due to the presence of a chiral centre at each metal centre in these complexes. The solid state structures of complexes 2b–c and 4a were determined by single crystal X-ray crystallography and showed that each bidentate arm of these multitopic ligands coordinates to the Rh or Ir centre in a bidentate fashion via the pyrazolyl-N2 and 1,2,3-triazolyl N3′ donors. The intermetallic distances in these solid state structures vary from 8.66 Å to 15.17 Å. These complexes were assessed as catalysts for the dihydroalkoxylation of alkynes using the cyclisation of 2-(5-hydroxypent-1-ynyl)benzyl alcohol, S, to a mixture of two spiroketals, 2,3,4,5-tetrahydro-spirol[furan-2,3′-isochroman], P1, and 3′,4′,5′,6′-tetrahydro-spiro[isobenzofuran-1(3H),2′(2H)pyran], P2, as the model reaction. The Rh(I) complexes (2a–d), with the highest TOF of 2052 h−1 for complex 2d, were the most active catalysts when compared with the other complexes under investigation here. The Ir(I) complexes (3a–d) were moderately active as catalysts for the same transformation. No significant enhancement in catalytic reactivity was observed with the Rh(I) series bi- and trimetallic complexes (2a–d) when compared with their monometallic analogues. The bi- and trimetallic Ir(I) complexes (3a–d) were much more efficient as catalysts for this transformation than their monometallic analogues, suggesting some intermetallic cooperativity. Rh(III), 4a–d, and Ir(III), 5a–d, complexes were not active as catalysts for this transformation.

 Please wait while we load your content...
Please wait while we load your content...