Methanetrisamidines in coordination chemistry – syntheses, structures and CH–NH tautomerism†
Abstract
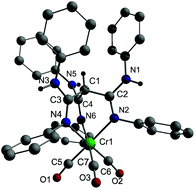
Methanetrisamidines {HC[C(NR)NHR]3} (R = i-Pr 1a; Ph 1b) were reacted with different metal complexes. Reaction of 1a with NiCl2(H2O)6 occurred with protonation of 1a and formation of {[C(C(NHi-Pr)2)3]2+[NiCl4]2−} 2, whereas the reaction with CuCl gave [C(C(N(i-Pr)CuCl)NHi-Pr)2(C(NHi-Pr)2)] 3. The formation of 2 and 3, which contain the N–H tautomeric form of 1a, occurred with H-migration from carbon to nitrogen. In contrast, reactions of 1b with [M(NCMe)3(CO)3] (M = Cr, Mo, W) yielded octahedral complexes fac-[M(CO)3CH(C(NHPh)NPh)3] (M = Cr 4a, Mo 4b, W 4c), in which the C–H tautomeric form is preserved. 1b is a rather strong σ-donor ligand as was shown by IR spectroscopy. The structures of 2, 3 and 4a were determined by single crystal X-ray diffraction.

 Please wait while we load your content...
Please wait while we load your content...