Synthesis, characterization and reactivity of an imidazolin-2-iminato aluminium dihydride†
Abstract
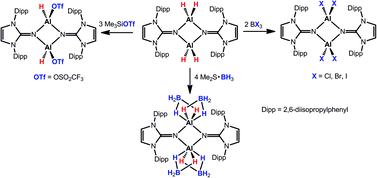
The reaction of bis(2,6-diisopropylphenyl)imidazolin-2-imine (LH, 1) with Me3N·AlH3 furnishes {μ-LAlH2}2 (2). The marked tendency of 2 to release its hydride substituents is ascribed to the strong electron-donor character of the imidazolin-2-iminato ligand. This is supported by its reactivity study and DFT calculations. In fact, compound 2 was further converted with Me3SiOTf, Me2S·BH3, Me2S·BBr3, and BX3 (with X = Cl, Br, and I) into {μ-LAl(H)OTf}2 (3), {μ-LAl(BH4)2}2 (4), and {μ-LAlX2}2 (5, X = Br; 6, X = Cl; 7, X = I), respectively. For all new aluminium complexes the formulation as dimers was evidenced by high resolution mass spectrometry, as well as single-crystal X-ray diffraction analysis. A prominent structural motif of these compounds is the square-planar four-membered Al2N2 ring with two bridging bulky imidazolin-2-imino moieties.
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...