Copper(ii) and triphenylphosphine copper(i) ethylene glycol carboxylates: synthesis, characterisation and copper nanoparticle generation†‡
Abstract
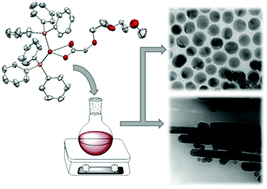
Ethylene glycol-functionalised copper(II) carboxylates Cu[O2CCR2(OC2H4)nOCH3]2 (n = 0–3; R = H, Me) (2a–e) have been prepared by the reaction of [Cu2(OAc)4·2H2O] with CH3O(C2H4O)nCR2CO2H (1a–e). Upon reduction of 2a–e with triphenylphosphine, the corresponding tris(triphenylphosphine)copper(I) complexes 3a–e were obtained, which could be converted to the bis(triphenylphosphine)copper(I) complexes 4a–e by removal of one phosphine ligand. Based on IR spectroscopy and single crystal X-ray structure analysis the binding motif of the carboxylato group on the copper ion is discussed. DSC, TG and TG-MS experiments were performed to analyse the thermal decomposition mechanism of 2–4. Complex 4c was used as a precursor for the generation of copper nanoparticles by thermal decomposition in hexadecylamine without the need of any further reactants. Depending on the precursor concentration, spherical copper nanoparticles with a mean diameter ranging from 10 to 85 nm as well as nanorods with a length of up to 1.3 μm (aspect ratios ranging between 2 and 32) were obtained. Electron diffraction analysis of the rods suggested that they consist of five domains which are arranged around a fivefold rotational axis.

 Please wait while we load your content...
Please wait while we load your content...