Fluorescent azobenzenes and aromatic aldimines featuring an N–B interaction
Abstract
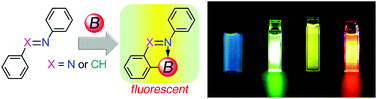
Azobenzenes are constituents of the commonly and widely used azo dyes. Many dyes, except for the azo dyes, have been utilized for fluorescent materials. However, there are only a few fluorescent azobenzene derivatives and their fluorescence efficiencies are quite low. The current perspective provides an account of the fluorescent azobenzenes and aromatic aldimines featuring an N–B interaction. Incorporation of the intramolecular N–B interaction by using the bis(pentafluorophenyl)boryl group makes the azobenzenes and aromatic aldimines fluorescent with a range of colours. Some of them fluoresce with extraordinarily high fluorescence quantum yields. Their synthesis, structures, fluorescence properties, and applications are discussed.
- This article is part of the themed collection: Coordination Programming: Science of Molecular Superstructures Towards Chemical Devices

 Please wait while we load your content...
Please wait while we load your content...