Effects of boron doping on the structural and optoelectronic properties of 9,10-diarylanthracenes†
Abstract
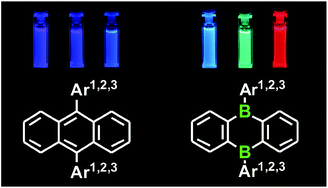
Key structural and optoelectronic properties of 9,10-dihydro-9,10-diboraanthracene (DBA) derivatives carrying mesityl (2a), 2-methylnaphthyl- (2b) and 9-phenyl-2,7-di-tert-butylanthryl (2c) substituents at the boron atoms have systematically been compared with the properties of their all-carbon congeners 4a–c. The experimental investigations have been augmented by quantum-chemical calculations. Steric repulsion leads to large dihedral angles between the aryl substituents and the DBA (2a–c) or anthrylene (4a–c) cores; as a result, the B–C bonds of 2a–c are kinetically shielded from hydrolysis and oxidative degradation. Lithium metal reduces the mesityl derivative 2a to the inverse sandwich complexes [Li(OR2)n]2[2a] (X-ray crystallography; OR2 = THF, n = 2; Et2O, n = 1). In line with the nodal structures of the LUMO of 2a/HOMO of [Li(THF)2]2[2a], the C–C bond lengths of the anionic fragment [2a]2− show characteristic differences to those of 2a and come close to the C–C bond lengths of the isoelectronic species 4a. X-ray crystallography on anti-2b × 2 C6H6 and anti-4b × 2 C6H6 reveals an essentially identical packing of the main molecules. The benzene solvate molecules, however, interact in a very different manner with anti-2b or anti-4b, which can be traced down to subtle disparities between the electron density distributions of the two compounds. 2a–c undergo a photoinduced aryl-to-DBA charge transfer; the back electron transfer results in blue (2a), green (2b) and red (2c) emission, albeit with low quantum yields. 4a–c are characterised by a local π–π* photoexcitation of the central 9,10-anthrylene fragments and corresponding blue emission. Each of the compounds 2a–c gives rise to two reversible DBA-centred one-electron transitions in the cyclic voltammogram.

 Please wait while we load your content...
Please wait while we load your content...