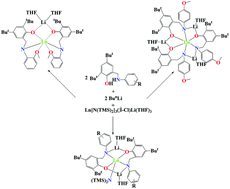
Electronic properties of the aminophenolate groups have obvious effect on the synthesis of aminophenolate lanthanide–lithium complexes. Amine elimination reactions of Ln[N(SiMe3)2]3(μ-Cl)Li(THF)3 with lithium aminophenolates [ArNHCH2(3,5-tBu2C6H2-2-O)Li(THF)]2 (Ar = p-ClC6H4, [ONH]Cl-p; p-BrC6H4, [ONH]Br-p) in tetrahydrofuran (THF) in a 1 : 2 molar ratio gave the bimetallic lanthanide–lithium amido complexes [NO]Cl-p2Ln[N(SiMe3)2][Li(THF)]2 (Ln = Y (1), Yb (2)), and [NO]Br-p2Ln[N(SiMe3)2][Li(THF)]2 (Ln = Y (3), Yb (4)). When the Ar groups are p-MeOC6H4, ([ONH]MeO-p) and o-MeOC6H4 ([ONH]MeO-o), similar reactions generated the homoleptic lanthanide–lithium complexes [NO]MeO-p3Ln[Li(THF)]3 (Ln = Y (5), Yb (6)) and [NO]MeO-o2Ln[Li(THF)] (Ln = Y (7), Yb (8)) in high isolated yields, respectively. Whereas the bimetallic lanthanide–lithium amido complexes [NO]Cl-o2Ln[N(SiMe3)2][Li(THF)]2 (Ln = Y (9), Yb (10)) can be obtained in good yields, when the Ar group is o-ClC6H4 ([ONH]Cl-o). All of these complexes were well characterized. X-ray structure determination revealed that these complexes have solvated monomeric structures. In complexes 1–4, 9, and 10, the lanthanide atom is five-coordinated by two oxygen atoms and two nitrogen atoms from two aminophenoxy ligands and one nitrogen atom from N(SiMe3)2 group to form a distorted trigonal bipyramidal geometry, whereas in complexes 5–8, the central lanthanide atom is six-coordinated by oxygen atoms, and nitrogen atoms from the aminophenoxy ligands to form a distorted octahedron. It was found that complexes 1–10 are highly efficient initiators for the ring-opening polymerization of 2,2-dimethyltrimethylene carbonate (DTC), affording the polymers with high molecular weights, and the homoleptic heterobimetallic lanthanide complexes showed apparently high activity.

 Please wait while we load your content...
Please wait while we load your content...