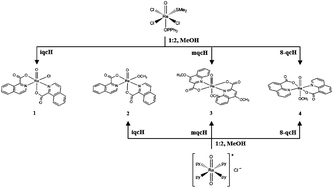
Six novel oxorhenium(V) complexes incorporating quinoline and isoquinoline carboxylic acid derivatives were prepared in good yields. Relying on the experimental conditions, compounds with two chelate ligands [ReOCl(iqc)2]·MeOH (1), [ReO(OMe)(iqc)2] (2), [ReO(OMe)(mqc)2] (3) and [ReO(OMe)(8-qc)2] (4) and compounds incorporating one bidentate ligand [ReOCl2(iqc)(PPh3)] (5) and [ReOCl2(mqc)(PPh3)] (6) were synthesized (iqcH = isoquinoline-1-carboxylic acid, mqcH = 4-methoxy-2-quinolinecarboxylic acid and 8-qcH = 8-quinolinecarboxylic acid). The reported compounds were characterized by spectroscopic methods and single crystal X-ray diffraction analysis. In compounds 1 and 2, one chelate ligand occupies an axial and an equatorial position, while the other one occupies two equatorial positions, forming a cis-(N,N) isomer. In turn, complexes 3 and 4 show a rare ligand arrangement with two trans-N, trans-O chelate ligands in the equatorial plane and a linear axial [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Re–OMe] unit. The complexes with one chelate ligand 5 and 6 are cis-(Cl,Cl)-isomers. All compounds were tested as potential catalysts in the epoxidation of cyclooctene with 3 equiv. of tert-butylhydroperoxide. The yield of cyclooctane oxide varies between 16 and 68% (50 °C, 24 h), and the catalytic competency of compounds 1–6 was discussed with regard to the structure of each complex.
Re–OMe] unit. The complexes with one chelate ligand 5 and 6 are cis-(Cl,Cl)-isomers. All compounds were tested as potential catalysts in the epoxidation of cyclooctene with 3 equiv. of tert-butylhydroperoxide. The yield of cyclooctane oxide varies between 16 and 68% (50 °C, 24 h), and the catalytic competency of compounds 1–6 was discussed with regard to the structure of each complex.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Re–OMe] unit. The complexes with one chelate
Re–OMe] unit. The complexes with one chelate 

 Please wait while we load your content...
Please wait while we load your content...