Tuning the metal binding site specificity of a fluorescent sensor protein: from copper to zinc and back†
Abstract
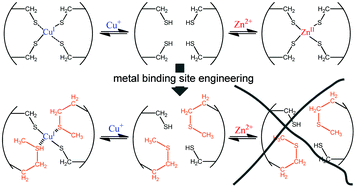
We previously reported the development of high affinity Zn2+ FRET sensors based on the Zn2+-mediated interaction between the CXXC motifs present in the copper chaperone
- This article is part of the themed collection: Bioinorganic chemistry

 Please wait while we load your content...
Please wait while we load your content...