Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light†
Abstract
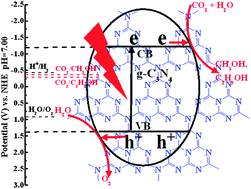
Two kinds of graphitic carbon nitride (g-C3N4) were synthesized through a pyrolysis process of urea or melamine. It is found that the obtained g-C3N4, as photocatalysts, can reduce CO2 to organic fuels under visible light, and exhibit different photoactivity and selectivity on the formation of CH3OH and C2H5OH. The product derived from the urea (denoted as u-g-C3N4) shows a mesoporous flake-like structure with a larger surface area and higher photoactivity for the CO2 reduction than the non-porous flaky product obtained from melamine (denoted as m-g-C3N4). Moreover, using u-g-C3N4 as a photocatalyst can result in the formation of a mixture containing CH3OH and C2H5OH, while m-g-C3N4 only leads to the selective formation of C2H5OH. The present interesting findings could shed light on the design of efficient, eco-friendly and convenient photocatalysts and the tuning of their photoreactivity in the field of sustainable light-to-energy conversion.

 Please wait while we load your content...
Please wait while we load your content...