Photocatalytic activities of Cu3xLa1–xTa7O19 solid solutions for H2 evolution under visible light irradiation†
Abstract
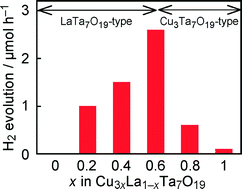
Development of new visible-light-driven oxide photocatalysts for H2 evolution was conducted with a concept of formation of solid solutions containing Cu(I) ions. Band gaps of Cu3xLa1–xTa7O19 solid solutions were 2.57–2.71 eV being remarkably narrower than that of LaTa7O19 (x = 0, 4.14 eV). The remarkable narrowing of the band gaps was due to the contribution of Cu 3d orbitals to valence bands. The solid solutions were capable of photocatalytic H2 evolution under visible light irradiation in the presence of methanol as an electron donor. Interestingly, the solid solutions with x = 0.2–0.8 showed higher activities than that of a native Cu(I)-tantalate, Cu3Ta7O19 (x = 0). Among the solid solutions, the x = 0.6 sample, Cu1.8La0.4Ta7O19, exhibited the highest activity. The apparent quantum yield of the optimized photocatalyst was 0.48% at 420 nm. Moreover, the good stability of Cu(I) ions in the solid solutions was proven through the investigation of photocatalytic properties and the XPS analysis. Thus, new visible-light-driven oxide photocatalysts capable of H2 evolution were developed by the formation of the Cu3xLa1–xTa7O19 solid solutions.

 Please wait while we load your content...
Please wait while we load your content...