Bulk gold (non-nanogold) catalysis of aerobic oxidations of amines, isocyanides, carbon monoxide, and carbene precursors
Abstract
Oxidative reactions that are catalysed by bulk gold consisting of ∼50 000 nm gold powder particles or gold (∼50 nm) supported on Al2O3 are reviewed. Using O2 as the oxidizing agent, bulk gold catalyzes the following types of reactions in organic ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHR], (2) the oxidation of
CHR], (2) the oxidation of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–R + H2N–R′ → R′–N
N–R + H2N–R′ → R′–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–R] and with
N–R] and with 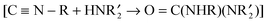 , (4) the reactions of CO with
, (4) the reactions of CO with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NHR)2], and (5) the reactions of diazoalkanes with
C(NHR)2], and (5) the reactions of diazoalkanes with 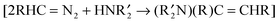 . In some of these reactions, amine oxides (e.g., Me3N–O) may be used in place of O2. Mechanisms of these and related reactions are examined. These studies show that bulk, non-nanogold is capable of catalysing a variety of different reactions.
. In some of these reactions, amine oxides (e.g., Me3N–O) may be used in place of O2. Mechanisms of these and related reactions are examined. These studies show that bulk, non-nanogold is capable of catalysing a variety of different reactions.

- This article is part of the themed collection: Towards environmentally benign catalytic oxidation

 Please wait while we load your content...
Please wait while we load your content...