Shape selectivity using ionic liquids for the preparation of silver and silver sulphide nanomaterials†
Abstract
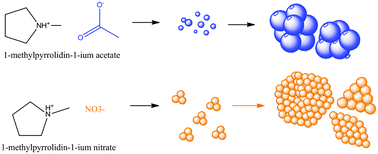
Electrodeposition of silver and silver sulphide was carried out from two protic ionic liquids. A change of the anion moiety of ionic liquid was found to bring about significant changes in the morphology of the nanocrystalline silver and silver sulphide deposits obtained. Effects of various parameters like deposition overpotential, change of the substrate, deposition time, etc. on the particle size and shape were studied. It was found that a change of anions of the ionic liquid from acetate to nitrate results in a wide difference in the morphology of the deposits obtained. Acetate containing ionic liquids result in globular nanocrystalline deposits whereas nitrate containing ionic liquids result in flat plates or sheets of silver deposits. Similar results were obtained for silver sulphide nanocrystals.

 Please wait while we load your content...
Please wait while we load your content...