Reversible addition of the OH radical to p-cymene in the gas phase: kinetic analysis assuming formation of a single adduct. Part 1
Abstract
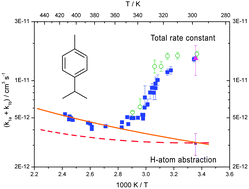
A flash photolysis-resonance fluorescence (FP-RF) technique was employed to study the kinetics and mechanism of the reaction of OH radicals with p-cymene at temperatures between 297 and 413 K in helium buffer gas. FP-RF experiments involved time-resolved detection of OH radicals by RF following vacuum-UV flash photolysis of H2O–p-cymene–He and H2O–He mixtures. Biexponential functions were fitted to decays of OH radicals according to reversible addition of OH radicals to p-cymene to form a single adduct. A rate constant of (15.7 ± 1.1) × 10−12 is obtained (in units of cm3 s−1) at room temperature (298 K) for the sum of the addition and abstraction channels (k1a + k1b) according to this simplified model. The Arrhenius plot reveals the step function typical of other aromatics and can be described using the expressions: 2 × 10−13 exp(+1300 K/T) at temperatures between 297 K and 324 K and 10−11 exp(−250 K/T) at temperatures between 345 K and 413 K. After consideration of the abstraction channel an equilibrium constant of k1a/k−1a = 6 × 10−26 exp(+9700 K/T) cm3 is obtained at temperatures between 297 and 325 K and 2 × 10−36 exp(+17 000 K/T) cm3 at temperatures between 325 and 380 K.

 Please wait while we load your content...
Please wait while we load your content...