Molecular dynamics of itraconazole at ambient and high pressure
Abstract
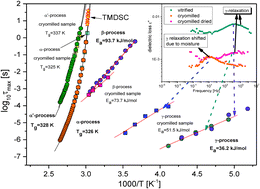
Comprehensive molecular dynamics studies of vitrified and cryogrounded itraconazole (Itr) were performed at ambient and elevated pressure. DSC measurements yielded besides melting and glass transition observed during heating and cooling of both samples two further endothermic events at around T = 363 K and T = 346 K. The nature of these transitions was investigated using X-ray diffraction, broadband dielectric spectroscopy and Density Functional Theory calculations. The X-ray measurements indicated that extra ordering in itraconazole is likely to occur. Based on calculations and theory derived by Letz et al.15 the transition observed at T = 363 K was discussed in the context of formation of the nematic mesophase. In fact, additional FTIR measurements revealed that order parameter variation in Itr shows a typical sequence of liquid crystal phases with axially symmetric orientational order; i.e. a nematic phase in the temperature range 361.7 K to 346.5 K and a smectic A phase below 346.5. Moreover, dielectric measurements demonstrated that except for the structural relaxation process, there is also slower mode above the glass transition temperature in both vitrified and cryogrounded samples. We considered the origin of this mode taking into account DFT calculations, rod like shape of itraconazole and distribution of its dipole moment vectors. For the dielectric data collected at elevated pressure, evolution of the steepness index versus pressure was determined. Finally, the pressure coefficient of the glass transition temperature was evaluated to be equal to 190 K GPa−1.

 Please wait while we load your content...
Please wait while we load your content...