Metal-free, polyether-mediated H2-release from ammonia borane: roles of hydrogen bonding interactions in promoting dehydrogenation†
Abstract
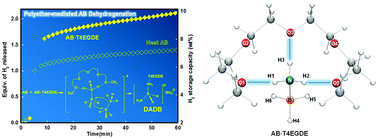
Polyetheral additives were found to be efficient promoters to enhance the rate of H2-release from ammonia borane (AB) at various temperatures. In particular, tetraethylene glycol dimethyl ether (T4EGDE, 29 wt% relative to AB + T4EGDE) exhibited significantly improved activities for AB dehydrogenation, with the material-based hydrogen storage capacity of 10.3 wt% at 125 °C within 40 min. In situ FT-IR spectroscopy indicated the formation of B-(cyclodiborazanyl)amino-borohydride (BCDB), borazine, and μ-aminodiborane as gaseous byproducts. In addition, 11B nuclear magnetic resonance (NMR) spectroscopy further revealed that diammoniate of diborane (DADB) was initially formed to give polyaminoborane as liquid and/or solid spent-fuel, consistent with previous reports. Density Functional Theory (DFT) calculations suggested that hydrogen bonding interactions between AB and a polyetheral promoter initially played an important role in increasing the reactivity of B–H bonds of AB by transferring electron density from oxygen atoms of the promoter into B–H bonds of AB. These partially activated, hydridic B–H bonds were proposed to help promote the formation of diammoniate of diborane (DADB), which is considered as a reactive intermediate, eventually enhancing the rate of H2-release from AB. In addition, our in situ solid state 11B magic angle spinning (MAS) NMR measurements further confirmed that the rate of DADB formation from AB with a small quantity of T4EGDE was found to be much faster than that of pristine AB even at 50 °C. This metal-free method for H2-release from AB with an added, small quantity of polyethers would be helpful to develop feasible hydrogen storage systems for long-term fuel cell applications.

 Please wait while we load your content...
Please wait while we load your content...