Cationic–anionic mediated charge compensation on La2Ti2O7 for visible light photocatalysis
Abstract
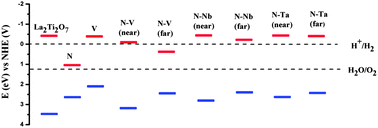
The cationic–anionic mediated charge compensation effect was studied in the layered perovskite La2Ti2O7 for the visible light photocatalysis. Our screened hybrid density functional study shows that the electronic structure of La2Ti2O7 can be tuned by the cationic (V, Nb, Ta)/anionic (N) mono- and co-doping. Such mono-doping creates impurity states in the band gap which helps the electron–hole recombination. But if the charge compensation is made by the cationic–anionic mediated co-doping then such impurity states can be removed and can be a promising strategy for visible light photocatalysis. The absolute band edge position of the doped La2Ti2O7 has been aligned with respect to the water oxidation/reduction potential. The calculated defect formation energy shows the stability of the co-doping system is improved due to the coulomb interactions and charge compensations effect.

 Please wait while we load your content...
Please wait while we load your content...