Oxidatively stable polyaniline:polyacid electrodes for electrochemical energy storage†
Abstract
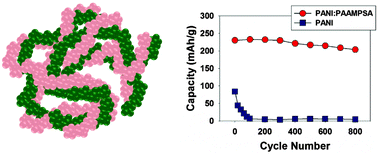
Conjugated polymers, such as polyaniline, have been widely explored as sensors, electrodes, and conductive fillers. As an electrode material in electrochemical energy storage systems, polyaniline can be subject to irreversible oxidation that reduces cycle life and electrode capacity, thus, limiting its widespread application. Here we present a simple route to produce and prepare polyaniline-based electrodes that are oxidatively stable up to 4.5 V vs. Li/Li+. The route uses a polyacid to stabilize the fully oxidized pernigraniline salt form of polyaniline, which is normally highly unstable as a homopolymer. The result is an organic electrode of exceptionally high capacity, energy density, power density, and cycle life. We demonstrate that the polyaniline:polyacid electrode stores 230 mA h g−1 of polyaniline for over 800 cycles, far surpassing homopolymer polyaniline under equivalent conditions. This approach provides a highly stable, electrochemically reversible replacement for conventional polyaniline.

 Please wait while we load your content...
Please wait while we load your content...