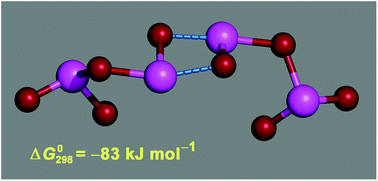
Biotic and abiotic emissions of molecular iodine and iodocarbons from the sea or the ice surface and the intertidal zone to the coastal/polar marine boundary layer lead to the formation of iodine oxides, which subsequently nucleate forming iodine oxide particles (IOPs). Although the link between coastal iodine emissions and ultrafine aerosol bursts is well established, the details of the nucleation mechanism have not yet been elucidated. In this paper, results of a theoretical study of a range of potentially relevant aggregation reactions of different iodine oxides, as well as complexation with water molecules, are reported. Thermochemical properties of these reactions are obtained from high level ab initio correlated calculations including spin–orbit corrections. The results show that the nucleation path most likely proceeds through dimerisation of I2O4. It is also shown that water can hinder gas-to-particle conversion to some extent, although complexation with key iodine oxides does not remove enough of these to stop IOP formation. A consistent picture of this process emerges from the theoretical study presented here and the findings of a new laboratory study reported in the accompanying paper (Gomez Martin et al., 2013).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...