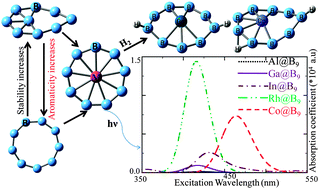
DFT calculations have been carried out to shed light on the electronic structure, optical properties and hydrogen adsorption capability of neutral MB9 (where M = Li3, Na3, K3, Al, Ga, In, Rh and Co) clusters. Electronic structural studies on the parent B93− clusters reveal that a less aromatic hypervalent B-centred B8 ring geometry is energetically more favoured. However, GM3+ (Al, Ga and In) in GM@B9 prefers a highly aromatic metal-centred 9-membered molecular wheel structure. In addition, the larger size of indium breaks the D9h symmetry of the molecular wheel by coming slightly out of the ring plane unlike Al@B9 and Ga@B9. B9n− (n = 1–3) is also neutralized with ‘n’ number of Li, Na and K which revealed that AM2B9 and AM2B9− result in pyramidal geometries while AM3B9 results in an irregular shape by retaining the B9 framework similar to the most stable conformer of B93−. Metal@B9 molecular wheels show optical absorption in a broad range of the spectrum (from UV to NIR: 260–1000 nm), which is attributed to the metal's ability to perturb the ring centred excited state. Ga@B9 and Co@B9 bind with hydrogen molecules in a dissociative manner, forming two covalent bonds with peripheral B atoms of the B9 ring.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...