Role of the surface–subsurface interlayer interaction in enhancing oxygen hydrogenation to water in Pd3Co alloy catalysts
Abstract
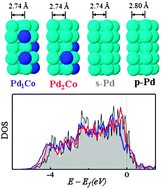
Based on density functional theory calculations, we present mechanisms underlying the improvement in the catalytic performance of Pd-based alloys for oxygen
- This article is part of the themed collection: Interfacial Phenomena in (De)hydrogenation Reactions

 Please wait while we load your content...
Please wait while we load your content...