Irreversible thermochromism in copper chloride Imidazolium Nanoparticle Networks†
Abstract
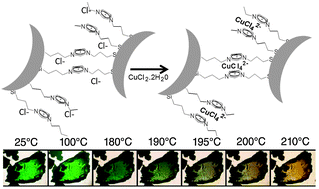
In this work Imidazolium
* Corresponding authors
a Vienna University of Technology, Institute of Materials Chemistry, 1060 Vienna, Austria
b University of Vienna, Faculty of Physics, 1090 Vienna, Austria
c
Interdisciplinary Laboratory on nanometric and supramolecular organization (LIONS), CEA Saclay, DSM, IRAMIS, SIS2M, 91191 Gif-sur-Yvette Cedex, France
E-mail:
marie-alexandra.gauthey-neouze@cea.fr
Fax: +43 158801 165988
Tel: +43 158801 165206
In this work Imidazolium

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
M. Kronstein, K. Kriechbaum, J. Akbarzadeh, H. Peterlik and M. Neouze, Phys. Chem. Chem. Phys., 2013, 15, 12717 DOI: 10.1039/C3CP50430A
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. You can use material from this article in other publications without requesting further permissions from the RSC, provided that the correct acknowledgement is given.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
