DFT based study of transition metal nano-clusters for electrochemical NH3 production†
Abstract
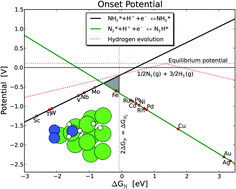
Theoretical studies of the possibility of producing ammonia electrochemically at ambient temperature and pressure without direct N2 dissociation are presented. Density functional theory calculations were used in combination with the computational standard hydrogen electrode to calculate the free energy profile for the reduction of N2 admolecules and N adatoms on transition metal nanoclusters in contact with an acidic electrolyte. This work has established linear scaling relations for the dissociative reaction intermediates NH, NH2, and NH3. In addition, linear scaling relations for the associative reaction intermediates N2H, N2H2, and N2H3 have been determined. Furthermore, correlations between the adsorption energies of N, N2, and H have been established. These scaling relations and the free energy corrections are used to establish volcanoes describing the onset potential for electrochemical ammonia production and hence describe the potential determining steps for the electrochemical ammonia production. The competing hydrogen evolution reaction has also been analyzed for comparison.

 Please wait while we load your content...
Please wait while we load your content...