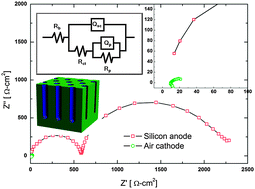
The mechanism of discharge termination in silicon–air batteries, employing a silicon wafer anode, a room-temperature fluorohydrogenate ionic liquid electrolyte and an air cathode membrane, is investigated using a wide range of tools. EIS studies indicate that the interfacial impedance between the electrolyte and the silicon wafer increases upon continuous discharge. In addition, it is shown that the impedance of the air cathode–electrolyte interface is several orders of magnitude lower than that of the anode. Equivalent circuit fitting parameters indicate the difference in the anode–electrolyte interface characteristics for different types of silicon wafers. Evolution of porous silicon surfaces at the anode and their properties, by means of estimated circuit parameters, is also presented. Moreover, it is found that the silicon anode potential has the highest negative impact on the battery discharge voltage, while the air cathode potential is actually stable and invariable along the whole discharge period. The discharge capacity of the battery can be increased significantly by mechanically replacing the silicon anode.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...