Construction of porous Mn(ii)-based metal–organic frameworks by flexible hexacarboxylic acid and rigid coligands†
Abstract
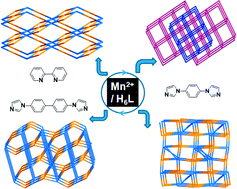
Solvothermal reactions of hexa[4-(carboxyphenyl)oxamethyl]-3-oxapentane (H6L) with rigid coligands, 2,2′-bipyridine (bipy), 1,4-bis-(1-imidazolyl)benzene (dib), and 4,4′-bis(1-imidazolyl)biphenyl (bibp) in the presence of manganese salts produce four porous Mn(II) metal–organic frameworks (Mn–MOFs), namely, Mn3(L)(bipy)2 (1), Mn3(L)(dib) (2), Mn3(L)(dib)(H2O)2 (3), and Mn6(L)2(bibp)1.5(H2O)54. Single crystal X-ray diffraction analyses reveal that 1–4 consist of trinuclear MnII subunits, which are further connected into four interesting nets, 4,4-connected pts net for 1, 4,6-connected fsc net for 2, and two novel nets for 3 and 4, by the synergistic effect of carboxylate groups and functionally complementary N-donor ligands. They exhibit high potential solvent accessible volumes of 35.0%, 33.5%, 45.1%, and 56.5% for 1–4, respectively. Magnetic studies indicate the presence of weak antiferromagnetic interactions between MnII centers in these compounds.

 Please wait while we load your content...
Please wait while we load your content...