New hydrogen-bond-aided supramolecular synthon: a case study of 2,4,6-trinitroaniline†
Abstract
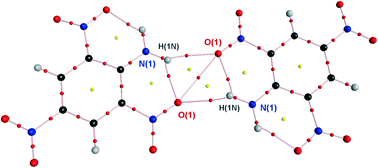
We have demonstrated that 2-nitroanilines and similar nitro compounds having a six-membered ring closed by an intramolecular H-bond tend to form centrosymmetric dimers in the crystalline phase. The probability of dimer formation is estimated to be 15% according to a database search in the Cambridge Structural Database. Bonding interactions in the dimer are found to be two O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N⋯H–N hydrogen bonds and an O⋯O interaction, with the total energy adding up to ca. 6 kcal mol−1. The bonding nature of interactions was proven by means of topological analysis of the experimental charge density obtained from a high-resolution single crystal X-ray diffraction experiment as well as from theoretical results of DFT and MP2 calculations.
N⋯H–N hydrogen bonds and an O⋯O interaction, with the total energy adding up to ca. 6 kcal mol−1. The bonding nature of interactions was proven by means of topological analysis of the experimental charge density obtained from a high-resolution single crystal X-ray diffraction experiment as well as from theoretical results of DFT and MP2 calculations.

 Please wait while we load your content...
Please wait while we load your content...