Racemic mixtures of chlorthalidone (CTD) have been structurally elucidated in three crystal forms (space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), namely, Form I, Form III (both true polymorphs) and a chloroform solvate. Here, we have structurally characterized the Form II, which, in contrast to the known forms, individual crystals are pure enantiomers. However, the Form II bulk product from spontaneous chiral resolution upon crystallization contains a mixture of crystals as a conglomerate, half of which consists solely of the (R)-enantiomer, the other half the (S)-enantiomer. Form II was solved in the space group P21 with one (R)-enantiomer unit in its asymmetric unit resembling that of Form III in terms of the chlorobenzenesulfonamide moiety conformation. Even though the (R)-enantiomer has been chosen for the single-crystal structure determination experiment, chiral chromatography and optical rotation data have inferred the conglomerate nature of Form II. Concerning the crystal packing of Form II, all hydrogen bonding functionalities of the sulfonamide group are saturated into the assembly of helical ribbons growing along the [010] direction, a supramolecular motif which is not found in the known crystal phases of the drug. Moreover, Form II shows packing similarity to the chloroform solvate mainly because the hydrogen bonding patterns of their isoindolinyl functionalities. The isoindolinyl stacking also occurs similarly in Form II and in the solvate form, with bends between the planes calculated through those moieties of stacked molecules of ca. 28° and 24°. In summary, we believe that the structural knowledge of Form II concerns new outlooks not only into the solid-state properties of CTD but also into its medicinal chemistry, e.g., regarding pharmacological profiles of the conglomerate.
), namely, Form I, Form III (both true polymorphs) and a chloroform solvate. Here, we have structurally characterized the Form II, which, in contrast to the known forms, individual crystals are pure enantiomers. However, the Form II bulk product from spontaneous chiral resolution upon crystallization contains a mixture of crystals as a conglomerate, half of which consists solely of the (R)-enantiomer, the other half the (S)-enantiomer. Form II was solved in the space group P21 with one (R)-enantiomer unit in its asymmetric unit resembling that of Form III in terms of the chlorobenzenesulfonamide moiety conformation. Even though the (R)-enantiomer has been chosen for the single-crystal structure determination experiment, chiral chromatography and optical rotation data have inferred the conglomerate nature of Form II. Concerning the crystal packing of Form II, all hydrogen bonding functionalities of the sulfonamide group are saturated into the assembly of helical ribbons growing along the [010] direction, a supramolecular motif which is not found in the known crystal phases of the drug. Moreover, Form II shows packing similarity to the chloroform solvate mainly because the hydrogen bonding patterns of their isoindolinyl functionalities. The isoindolinyl stacking also occurs similarly in Form II and in the solvate form, with bends between the planes calculated through those moieties of stacked molecules of ca. 28° and 24°. In summary, we believe that the structural knowledge of Form II concerns new outlooks not only into the solid-state properties of CTD but also into its medicinal chemistry, e.g., regarding pharmacological profiles of the conglomerate.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), namely, Form I, Form III (both true polymorphs) and a chloroform solvate. Here, we have structurally characterized the Form II, which, in contrast to the known forms, individual crystals are pure enantiomers. However, the Form II bulk product from spontaneous chiral resolution upon
), namely, Form I, Form III (both true polymorphs) and a chloroform solvate. Here, we have structurally characterized the Form II, which, in contrast to the known forms, individual crystals are pure enantiomers. However, the Form II bulk product from spontaneous chiral resolution upon 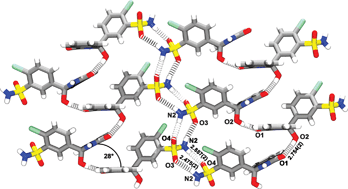

 Please wait while we load your content...
Please wait while we load your content...