Synthesis of γ-labeled nucleoside 5′-triphosphates using click chemistry†
Abstract
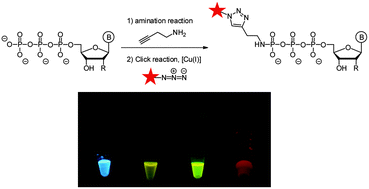
Real-time enzymatic studies are gaining importance as their chemical and technical instrumentation improves. Here we report the efficient synthesis of γ-alkyne modified triphosphate amidates that are converted into a variety of γ-fluorophore labeled triphosphates by Cu(I) catalyzed alkyne/azide click reactions. The synthesized triphosphates are incorporated into DNA by DNA polymerases.

 Please wait while we load your content...
Please wait while we load your content...