Selective fluorometric detection of aromatic thiols by a chemosensor containing two electrophilic sites with different local softness†
Abstract
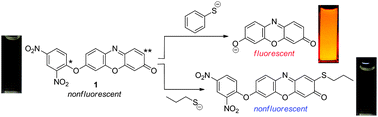
A resorufin–dinitrophenyl ether conjugate (1) shows emission enhancement for aromatic thiols in aqueous media with a neutral–basic pH, while being nonemissive for aliphatic thiols. This is achieved by two electrophilic sites with different local softness on compound 1; the respective sites selectively react with aromatic or aliphatic thiolate anions.

 Please wait while we load your content...
Please wait while we load your content...