Hypervalent iodine-mediated oxidative cyclisation of p-hydroxy acetanilides to 1,2-dispirodienones†
Abstract
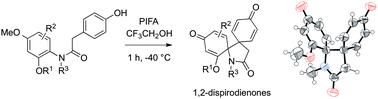
1,2-Dispirodienones were synthesized by hypervalent iodine-mediated phenolic oxidation of p-hydroxy acetanilides. The reaction is compatible with several substituted anilides and affords a new class of 1,2-dispirodienones that are remarkably stable under thermal or acidic conditions.

 Please wait while we load your content...
Please wait while we load your content...