Epoxidation of bromoallenes connects red algae metabolites by an intersecting bromoallene oxide – Favorskii manifold†
Abstract
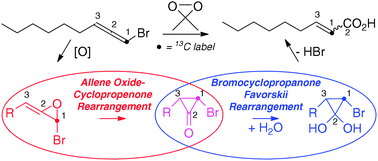
DMDO epoxidation of bromoallenes gives directly α,β-unsaturated carboxylic acids under the reaction conditions. Calculated (ωB97XD/6-311G(d,p)/SCRF = acetone) potential energy surfaces and 2H- and 13C-labeling experiments are consistent with bromoallene oxide intermediates which spontaneously rearrange via a bromocyclopropanone in an intersecting bromoallene oxide – Favorskii manifold.

 Please wait while we load your content...
Please wait while we load your content...