Trichloromethyl ketones: asymmetric transfer hydrogenation and subsequent Jocic-type reactions with amines†
Abstract
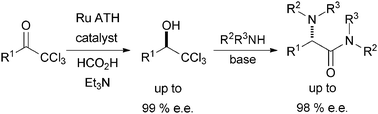
Amino-amides are important pharmaceutical building-blocks. The enantioselective reduction of trichloromethyl ketones using ruthenium transfer hydrogenation catalysts is reported. The products react in a range of Jocic-type reactions to give enantiomerically enriched amino-amides.

 Please wait while we load your content...
Please wait while we load your content...