An efficient synthesis of trisubstituted oxazoles via chemoselective O-acylations and intramolecular Wittig reactions†
Abstract
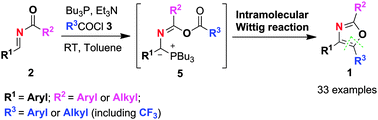
Preparation of new types of trisubstituted oxazoles is realized via chemoselective O-acylations and intramolecular Wittig reactions with ester functionalities using in situ formed phosphorus ylides as key intermediates. A plausible reaction mechanism for this undiscovered chemistry is also proposed based on the existence of expected and rearranged isomeric oxazoles.

 Please wait while we load your content...
Please wait while we load your content...