Rapid microfluidic flow hydrogenation for reduction or deprotection of 18F-labeled compounds†
Abstract
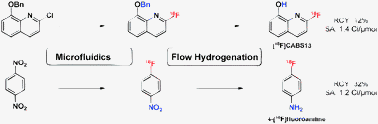
We have combined the benefits of both microfluidics and flow
- This article is part of the themed collection: Recent Advances in Flow Synthesis and Continuous Processing

 Please wait while we load your content...
Please wait while we load your content...