Reductive amination of tertiary anilines and aldehydes†
Abstract
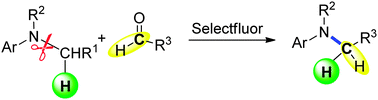
An unprecedented oxidant-mediated reductive amination of tertiary anilines and aldehydes without external reducing agents was developed via the nucleophilic attack of the oxygen atom of the carbonyl group to in situ generated iminium ions, in which tertiary anilines were used as both nitrogen source and reducing agent for the first time.

 Please wait while we load your content...
Please wait while we load your content...