Synthesis, structure and reactivity of cyclopropenyl-1-ylidene stabilized S(ii), Se(ii) and Te(ii) mono- and dications†‡
Abstract
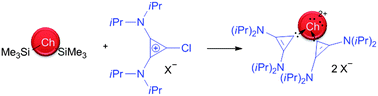
The syntheses and structural characterization of
- This article is part of the themed collection: Emerging Investigators 2013

 Please wait while we load your content...
Please wait while we load your content...