Sub-ppm quantification of Hg(ii) in aqueous media using both the naked eye and digital information from pictures of a colorimetric sensory polymer membrane taken with the digital camera of a conventional mobile phone†
Abstract
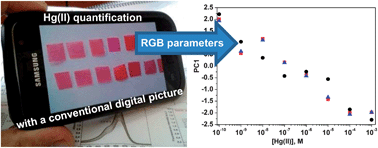
We present colorimetric sensory membranes for detecting Hg(II) in aqueous media. The colour response of the sensory materials can be tuned for detection with the naked eye, such as the maximum contaminant level of Hg(II) that is set by the United States Environmental Protection Agency (EPA) for drinking

 Please wait while we load your content...
Please wait while we load your content...