1,5-, 2,6- and 9,10-distyrylanthracenes as luminescent organic semiconductors†
Abstract
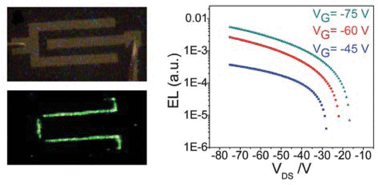
We report the synthesis, molecular and crystal structures, optoelectronic properties and organic field-effect transistor (OFET) studies of three isomeric distyrylanthracene derivatives 2,6-DPSAnt, 1,5-DPSAnt and 9,10-DPSAnt. The analysis of the structure–property relationships reveals that π-conjugation in this series is defined by two competing factors: the co-planarity of the styryl substituents (highest in 2,6-DPSAnt) and the electronic communication through the anthracene core (strongest in 9,10-DPSAnt). The charge-transport properties of the compounds, on the other hand, are primarily controlled by the solid state packing which is more efficient for the linear 2,6-DPSAnt derivative. Accordingly, the highest field-effect mobility (up to 0.75 cm2 V−1 s−1) was measured for 2,6-DPSAnt. Unexpectedly high charge mobility of 1,5-DPSAnt (up to 0.15 cm2 V−1 s−1) was attributed to excellent thin-film morphology (nearly layer-by-layer growth) and favorable molecular packing in the films, different from that found in single crystals. Unfavorable molecular packing and poor film morphology observed for 9,10-DPSAnt predictably led to no transistor properties being observed for this isomer. Both 2,6-DPSAnt and 1,5-DPSAnt afforded light-emitting transistors with green light emission area localized near an electron-injecting electrode.

 Please wait while we load your content...
Please wait while we load your content...