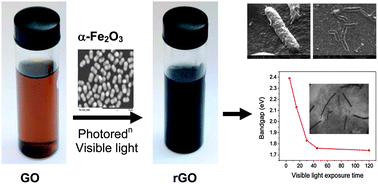
We report the synthesis of capsule-embedded reduced graphene oxide (rGO) by photocatalytic reduction in the presence of peanut shaped α-Fe2O3 particles under visible light. This process is able to produce highly stabilized water dispersible rGO without the help of a stabilizer, which is suitable to make thin films on a glass substrate by the drop casting method. In addition, the evolution of the morphology with different visible light exposure times ranging from 5 to 120 minutes reveals that the catalyst leads to transformation of a few rGO sheets into capsules via a core–shell structure. These capsules are stable on the rGO sheet at a vacuum of 10−9 Torr, but unstable under vacuum annealing at 400 °C and 10−5 Torr that created impressions on the rGO film. The bandgap of the capsule-embedded rGO decreases non-linearly with the visible light exposure time; from 2.47 eV at 5 min to 1.73 eV at 120 min. The vacuum annealed rGO films on glass substrates show an electrical conductivity of 2000 S m−1 at 300 K and the conduction is dominated by two-dimensional variable range hopping. The formation of capsules is described based on electrostatic interactions in association with the dipole force of the monodispersed peanut shaped α-Fe2O3 particles, whereas, the bandgap opening and high conductivity are attributed to the residual oxygen functional groups such as carbonyl (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and epoxy (C–O–C) on the rGO, in addition to the modulated surface morphology.
O) and epoxy (C–O–C) on the rGO, in addition to the modulated surface morphology.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and epoxy (C–O–C) on the rGO, in addition to the modulated surface morphology.
O) and epoxy (C–O–C) on the rGO, in addition to the modulated surface morphology.

 Please wait while we load your content...
Please wait while we load your content...